Description
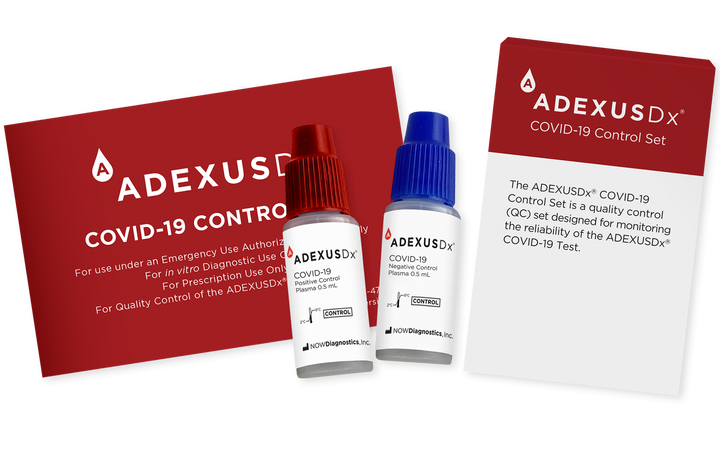
Negative controls were sourced from plasma donors. Positive controls are prepared by diluting pooled plasma, positive for antibodies to SARS-CoV-2, from plasma donors with negative control plasma to achieve a low positive. All plasma samples have been heat-inactivated, defibrinated, and clarified. A low concentration of ProClin™ preservative has been added to the resultant serum controls to extend their shelf life by effectively inhibiting a broad spectrum of microbes. Negative and positive controls are plasma samples, a typical patient sample used for ADEXUSDx® COVID-19 Test performance.
Negative controls were sourced from plasma donors. Positive controls are prepared by diluting pooled plasma, positive for antibodies to SARS-CoV-2, from plasma donors with negative control plasma to achieve a low positive. All plasma samples have been heat-inactivated, defibrinated, and clarified. A low concentration of ProClin™ preservative has been added to the resultant serum controls to extend their shelf life by effectively inhibiting a broad spectrum of microbes. Negative and positive controls are plasma samples, a typical patient sample used for ADEXUSDx® COVID-19 Test performance.
Specifications
*USAGE / SAFETY STATEMENT
The ADEXUSDx® COVID-19 Control Set is a quality control (QC) set designed for monitoring the reliability of the ADEXUSDx® COVID-19 Test. It is recommended that the ADEXUSDx® COVID-19 Control Set is tested to ensure proper performance of the test each time a new lot is used, when a new operator performs the test, when a new shipment of reagents is used, and to investigate the cause of repeated invalid results.
COUNTRY OF ORIGIN
United States
PRECAUTIONARY STATEMENTS
Negative control samples are sourced from human plasma donors who tested negative for HIV,
AIDS, Hepatitis B, and Hepatitis C.
All control samples have been heat-inactivated, defibrinated, and clarified. Heat activation of
samples was performed for 30 minutes at 65°C. DO NOT USE the controls if the droppers were not sealed.
DO NOT USE post the expiration date on the label. Expiration is indicated on the labeling as 30
days post shipment. Check the expiration date prior to use. Refer to CDC guidelines and local regulations for handling and disposal of positive and negative
controls.
REGULATORY NOTICE & RESTRICTIONS
Read the Product Insert completely before using the ADEXUSDx® COVID-19 Control Set. This product has not been FDA cleared or approved but has been authorized for emergency use by FDA under an EUA for use by authorized laboratories. This product is for use with a test authorized only for detecting the presence of total antibodies to
SARS-CoV-2, not for any other viruses or pathogens.
The emergency use of this product is only authorized for the duration of the declaration that
circumstances exist justifying the authorization of emergency use of in vitro diagnostics for
detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and
Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated or authorization is
revoked sooner.
QUALITY CERTIFICATION / COMPLIANCE
This product was manufactured in NOWDiagnostics, Inc. facilities based on a quality system compliant with GMP for Medical Devices and ISO 13485.
RESULT WINDOW
15-30 Minutes
SAMPLE TYPE
Human venous whole blood (dipotassium EDTA), plasma (dipotassium EDTA), serum, and fingerstick whole blood