Specifications
CLASS
Chiral Catalysts, Chiral Ligands, Chiral Reagents
SUBCLASS
Chiral Organocatalysts (Other)
APPLICATION
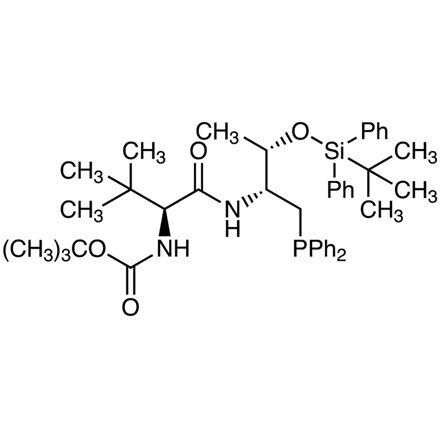
A Novel Dipeptide-Based Chiral Phosphine Organocatalyst for Asymmetric Cyclization
Typical procedure (entry 1: R = CH3CH2CH2-): To a dried round bottom flask is added N-butylidene-P,P-diphenylphosphinic amide (27.1 mg, 0.1 mmol), O-TBDPS-D-Thr-N-Boc-L-tert-Leu-diphenylphosphine (3.6 mg, 0.005 mmol) and MS5Å (60 mg) under N2, followed by the addition of anhydrous Et2O (1 mL). The reaction mixture is cooled to 0 °C, tert-butyl 2,3-butadienoate (22 micro-L, 0.15 mmol) is then added, and the mixture is stirred at 0 °C for 30 min. The reaction mixture is then filtered (to remove MS5Å) and concentrated under reduced pressure. The residue is purified by column chromatography on silica gel (eluent: hexane/EtOAc = 10:1 to 2:1) to afford the cycloaddition product (37.9 mg, 92% yield, 96% ee) as a colorless oil.
PUBCHEM SUBSTANCE ID
172089008.0
BEILSTEIN/REAXYS NUMBER
21221495.0
*USAGE / SAFETY STATEMENT
For laboratory research and development purposes only.
QUALITY CERTIFICATION / COMPLIANCE
Fukaya factory is certified for GMP, ISO14001:2004 (JQA-EM5386) and IQ Net (JP-JQA-EM5386).
APPEARANCE
White to Almost white powder to crystal
MOLECULAR FORMULA
C43H57N2O4PSi
PURITY (HPLC)
min. 98.0 area%
PURITY / ANALYSIS METHOD
>98.0%(HPLC)