APPLICATION
TCI Practical Example: Buchwald-Hartwig Amination Using Pd2(dba)3 and tBu3P・HBF4
Used Chemicals
4-Chloroanisole [C0122]
Diphenylamine
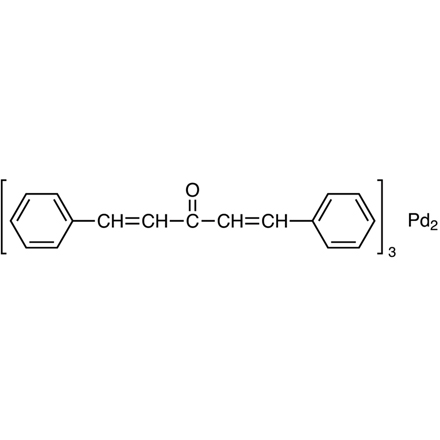
Tris(dibenzylideneacetone)dipalladium(0) (= Pd2(dba)3) [T2184]
Tri-tert-butylphosphonium Tetrafluoroborate (= tBu3P・HBF4) [T2584]
Sodium tert-Butoxide (= NaOtBu) [S0450]
Toluene
Procedure
To a 3-necked 300 mL round bottom flask was charged with diphenylamine (5.01 g, 29.6 mmol, 1.0 eq.), 4-chloroanisole (4.48 g, 31.4 mmol, 1.05 eq.) and degassed toluene (150 mL). To this solution was added Pd2(dba)3 (0.287 g, 0.131 mmol, 1 mol%), tri-tert-butylphosphonium tetrafluoroborate (0.198 g, 0.683 mmol, 2 mol%) and sodium tert-butoxide (6.34 g, 66.0 mmol, 2.2 eq.). The reaction mixture was refluxed for 16 hr under nitrogen atmosphere. After cooled to room temperature, the reaction was diluted with CH2Cl2 (300 mL). The suspension was filtered and the filtrate was dried over Na2SO4 and concentrated under reduced pressure to afford the crude and brown solid. The crude product was purified by silica-gel column chromatography (hexane/EtOAc = 99/1 then 8/1) to afford the light brown solid (7.0 g) containing 10 mol% of diphenylamine. Removal of the residual diphenylamine by recrystallization from hexane (55 mL, 60 °C then 15 °C) gave 4-methoxytriphenylamine as a white solid (5.26 g, 65 %).
Experimenter’s Comments
The reaction mixture was monitored by TLC (EtOAc/hexane = 1/10. Starting materials: Rf = 0.36 (diphenylamine), 0.59 (4-chloroanisole); target product: Rf = 0.46).
Analytical Data(4-Methoxytriphenylamine)
1H NMR (400 MHz, CD2Cl2); δ 7.26-7.17 (m, 4H), 7.10-6.98 (m, 6H), 6.98-6.91 (m, 2H), 6.89-6.82 (m, 2H), 3.79 (s, 3H).
13C NMR (101 MHz, CD2Cl2); δ 156.72, 148.56, 141.00, 129.39, 127.72, 123.12, 122.15, 115.05, 55.77.
Lead References
Air-Stable Trialkylphosphonium Salts: Simple, Practical, and Versatile Replacements for Air-Sensitive Trialkylphosphines. Applications in Stoichiometric and Catalytic Processes
M. R. Netherton, G. C. Fu, Org. Lett. 2001, 3, 4295.
An Air and Thermally Stable One- Component Catalyst for the Amination of Aryl Chlorides
D. Zim, S. L. Buchwald, Org. Lett. 2003, 5, 2413.
Other References
A Simple Catalytic Method for the Conversion of Aryl Bromides to Arylamines
A. Guram, R. Rennels, S. Buchwald, Angew. Chem. Int. Ed. 1995, 34, 1348.
Palladium-catalyzed synthesis of arylamines from aryl halides. Mechanistic studies lead to coupling in the absence of tin reagents
J. Louie, J. F. Hartwig, Tetrahedron Lett. 1995, 36, 3609.
Application
TCI Practical Example: Buchwald-Hartwig amination using Pd2(dba)3 and XPhos as catalysts
Used Chemicals
1,4-Dichlorobenzene [D0687]
Piperidin
Tris(dibenzylideneacetone)dipalladium(0) (= Pd2(dba)3) [T2184]
2-Dicyclohexylphosphino-2',4',6'-triisopropylbiphenyl (= XPhos) [D5038]
Sodium tert-Butoxide [S0450]
Toluene
Procedure
1,4-Dichlorobenzene (500 mg, 3.40 mmol) and piperidine (869 mg, 10.2 mmol) were dissolved in degassed toluene (15.0 mL). To this solution was added sodium tert-butoxide (817 mg, 8.50 mmol), XPhos (65 mg, 0.136 mmol, 4 mol%) and Pd2(dba)3 (47 mg, 0.051 mmol, 1.5 mol%). The reaction was refluxed for 16 h and quenched with H2O (15 mL) after cooling to room temperature. The organic phase was separated and washed with H2O (10 mL) and brine (10 mL), dried over Na2SO4 (30 g), concentrated under reduced pressure to afford the crude product as brown solid, which was purified by column chromatography on silica gel (CH2Cl2 100% → CH2Cl2: EtOAc = 30: 70) to afford the product 1 as a pale yellow solid. The obtained solid was dissolved in hexane and decolorized by activated carbon. After removal of activated carbon by filtration, the filtrate was concentrated to afford the product 1 as a white powder (782 mg, 2.66 mmol, 78%).
Experimenter's Comments
Toluene was degassed by refluxing under nitrogen atmosphere.
Completion of the reaction was confirmed by HPLC.
Obtained product was colorized gradually by the exposure of air and light.
Analytical Data(Compound 1)
1H NMR (400 MHz, CDCl3); δ 6.92 (s, 4H), 2.92 (t, J = 5.9, 8H), 1.54 (m, 8H), 1.32 (m, 4H).
Other References
A Simple Catalytic Method for the Conversion of Aryl Bromides to Arylamines
A. S. Guram, R. A. Rennels, S. L. Buchwald, Angew. Chem. Int. Ed. 1995, 34, 1348.
Palladium-catalyzed synthesis of arylamines from aryl halides. Mechanistic studies lead to coupling in the absence of tin reagents
J. Louie, J. F. Hartwig, Tetrahedron Lett. 1995, 36, 3609.
Application
TCI Practical Example: Suzuki-Miyaura coupling
Used Chemicals
4'-Chloroacetophenone [C0033]
1-Boc-1,2,3,6-tetrahydropyridine-4-boronic Acid Pinacol Ester [B4051]
Tris(dibenzylideneacetone)dipalladium(0) (= Pd2(dba)3) [T2184]
2-Dicyclohexylphosphino-2',6'-dimethoxybiphenyl (= SPhos) [D5036]
K3PO4
Toluene
Ethanol
H2O
Procedure
4'-Chloroacetophenone (200 mg, 1.29 mmol) and 1-Boc-1,2,3,6-tetrahydropyridine-4-boronic acid pinacol ester (440 mg, 1.42 mmol) were dissolved in degassed mixture of toluene (5.0 mL), EtOH (2.0 mL) and water (2.0 mL). To this solution was added K3PO4 (820 mg, 3.88 mmol), SPhos (21 mg, 0.052 mmol, 4 mol%) and Pd2(dba)3 (18 mg, 0.020 mmol, 1.5 mol%). The reaction mixture was refluxed for 21 h, allowed to cool to room temperature and quenched with H2O (5 mL). The organic phase was separated and washed with H2O (5 mL) and brine (5 mL), dried over Na2SO4 (10 g), concentrated under reduced pressure to afford the crude product as orange oil. It was purified by column chromatography on silica-gel (hexane 100% ~ hexane: EtOAc = 70: 30) to afford the product 1 as colorless crystalline solid (310 mg, 1.03 mmol, 80%).
Experimenter's Comments
Toluene was degassed by refluxing under nitrogen atmosphere.
Analytical Data(N-Phenylethyl-3-phenylpropaneamide)
1H NMR (400 MHz, benzene-d6); δ 7.90 (d, J = 8.7 Hz, 2H), 7.42 (d, J = 8.7 Hz, 2H), 6.15 (brs, 1H), 4.08 (m, 2H), 3.63 (m, 2H), 2.50 (s, 3H), 2.52 (m, 2H), 1.47 (s, 9H).
Application
Palladium Catalyst
C-C Formation Reaction