Specifications
SHIPPING NOTES
Blue Ice or 4ºC. All orders received in North America by 12:00 PM PST Monday to Thursday will usually be shipped out on the same day. North American customers will typically receive their orders within 1-2 business days. All shipments are F.O.B. from point of shipment, pre-paid and added to the invoice. Freight charges include but not limited to: priority service and packaging materials.Products are shipped with “cold packs” in insulated containers. There is a charge for this packaging and for the shipping costs via FedEx. Please inspect packages immediately upon receipt and inform Labscoop Customer Support of any shortage or damage within 48 hours of receipt. We can only accept returns after we have given prior authorization and issued specific shipping instructions. If a customer wishes to use their own FedEx account number please indicate this on the purchase order or during checkout.
APPLICATION
WB, IHC, ICC/IF
GENE ID
https://www.ncbi.nlm.nih.gov/gene/3315
*USAGE / SAFETY STATEMENT
Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only.
ANTIBODY TYPE
WB (1:1000), IHC (1:100), ICC/IF (1:200); optimal dilutions for assays should be determined by the user.
REACTIVITY SPECIES
Human | Mouse | Rat
PURIFICATION
Peptide Affinity Purified
QUALITY CERTIFICATION / COMPLIANCE
ISO 9001:2015
IMMUNOGEN
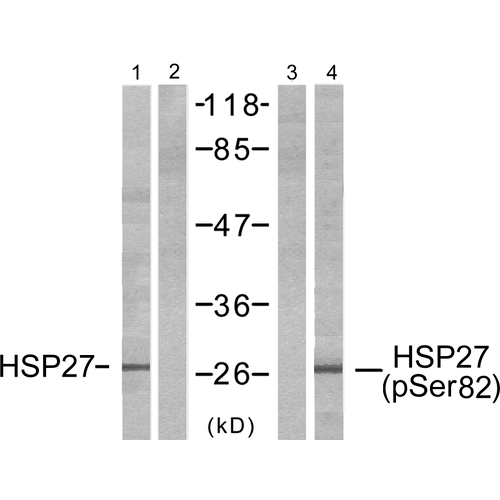
Synthesized phosphopeptide derived from human HSP27 around the phosphorylation site of serine15 (G-P-SP-W-D).
SPECIFICITY
Detects endogenous levels of HSP27only when phosphorylated at serine 15.
STORAGE BUFFER
PBS pH 7.4, 50% glycerol, 150mM NaCl, 0.02% sodium azide *Storage buffer may change when conjugated
ANTIBODY ID
https://antibodyregistry.org/search.php?q=AB_2712559
CELLUAR LOCALIZATIONS
Cytoplasm | Nucleus
TISSUE SPECIFICITY
Ubiquituous.
SCIENTIFIC BACKGROUND
HSP27s belong to an abundant and ubiquitous family of small heat shock proteins (sHSP). It is an important HSP found in both normal human cells and cancer cells. The basic structure of most sHSPs is a homologous and highly conserved amino acid sequence, with an α-crystallin domain at the C-terminus and the WD/EPF domain at the less conserved N-terminus. This N-terminus is essential for the development of high molecular oligomers (1, 2). HSP27-oligomers consist of stable dimers formed by as many as 8-40 HSP27 protein monomers (3). The oligomerization status is connected with the chaperone activity: aggregates of large oligomers have high chaperone activity, whereas dimers have no chaperone activity (4). HSP27 is localized to the cytoplasm of unstressed cells but can redistribute to the nucleus in response to stress, where it may function to stabilize DNA and/or the nuclear membrane. Other functions include chaperone activity (as mentioned above), thermo tolerance in vivo, inhibition of apoptosis, and signal transduction. Specifically, in vitro, it acts as an ATP-independent chaperone by inhibiting protein aggregation and by stabilizing partially denatured proteins, which ensures refolding of the HSP70 complex. HSP27 is also involved in the apoptotic signaling pathway because it interferes with the activation of cytochrome c/Apaf-1/dATP complex, thereby inhibiting the activation of procaspase-9. It is also hypothesized that HSP27 may serve some role in cross-bridge formation between actin and myosin (5). And finally, HSP27 is also thought to be involved in the process of cell differentiation. The up-regulation of HSP27 correlates with the rate of phosphorylation and with an increase of large oligomers. It is possible that HSP27 may play a crucial role in termination of growth (6). Looking for more information on HSP27? Visit our new HSP27 Scientific Resource Guide at http://www.HSP27.com.
REFERENCES
1. Kim K.K., Kim R., and Kim, S. (1998) Nature 394(6693): 595-599.
2. Van Montfort R., Slingsby C., and Vierling E. (2001) Addv Protein Chem. 59: 105-56.
3. Ehrnsperger M., Graber S., Gaestel M. and Buchner J. (1997) EMBO J. 16: 221-229.
4. Ciocca D.R., Oesterreich S., Chamness G.C., McGuire W.L., and Fugua S.A. (1993) J Natl Cancer Inst. 85 (19): 1558-70.
5. Sarto C., Binnz P.A., and Mocarelli P. (2000) Electrophoresis. 21(6): 1218-26.
6. Arrigo A.P. (2005) J Cell Biochem. 94(2): 241-6.
RESEARCH AREA
Cell Signaling | Protein Trafficking | Chaperone Proteins | Cell Signaling | Cytoskeleton | Microfilaments | Actin | Actin Assembly | Cell Signaling | Cytoskeleton | Microtubules | Cancer | Tumor Biomarkers | Cardiovascular System | Atherosclerosis | Cardiovascular System | Heart | Contractility |Cancer | Heat Shock
ALTERNATIVE NAMES
28kDa heat shock protein Antibody, CMT2F Antibody, HSP25 Antibody, HSP27 Antibody, HSP28 Antibody, HSPB1 Antibody, SRP27 Antibody