Specifications
SHIPPING NOTES
Blue Ice or 4ºC. All orders received in North America by 12:00 PM PST Monday to Thursday will usually be shipped out on the same day. North American customers will typically receive their orders within 1-2 business days. All shipments are F.O.B. from point of shipment, pre-paid and added to the invoice. Freight charges include but not limited to: priority service and packaging materials.Products are shipped with “cold packs” in insulated containers. There is a charge for this packaging and for the shipping costs via FedEx. Please inspect packages immediately upon receipt and inform Labscoop Customer Support of any shortage or damage within 48 hours of receipt. We can only accept returns after we have given prior authorization and issued specific shipping instructions. If a customer wishes to use their own FedEx account number please indicate this on the purchase order or during checkout.
APPLICATION
WB, ICC/IF, IP
GENE ID
https://www.ncbi.nlm.nih.gov/gene/811999
*USAGE / SAFETY STATEMENT
Not for use in humans. Not for use in diagnostics or therapeutics. For in vitro research use only.
ANTIBODY TYPE
WB (1:2000), ICC/IF (1:50); optimal dilutions for assays should be determined by the user.
REACTIVITY SPECIES
Plasmodium falciparum
PURIFICATION
Protein A Purified
QUALITY CERTIFICATION / COMPLIANCE
ISO 9001:2015
IMMUNOGEN
Recombinant full length PfHSP90
SPECIFICITY
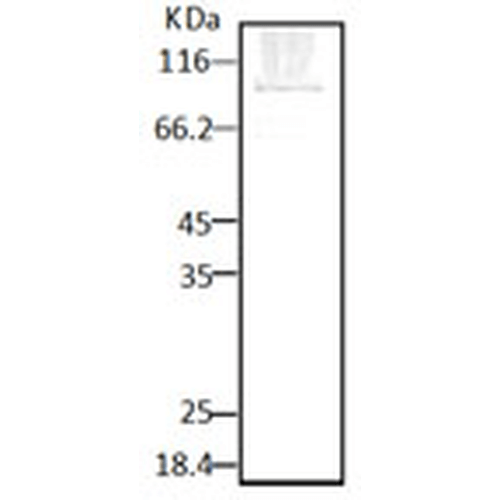
Detects ~ 86kDa. Specific to P. falciparum and does not cross-react to HSP90 from Human, yeast, and dictyostelium.
STORAGE BUFFER
PBS pH7.4, 50% glycerol, 0.09% sodium azide *Storage buffer may change when conjugated
ANTIBODY ID
https://antibodyregistry.org/search.php?q=AB_2704038
CELLUAR LOCALIZATIONS
Cytoplasm | Melanosome
SCIENTIFIC BACKGROUND
HSP90 is an abundantly and ubiquitously expressed heat shock protein. It is understood to exist in two principal forms α and β, which share 85% sequence amino acid homology. The two isoforms of HSP90 are expressed in the cytosolic compartment (1). Despite the similarities, HSP90α exists predominantly as a homodimer while HSP90β exists mainly as a monomer.(2) From a functional perspective, HSP90 participates in the folding, assembly, maturation, and stabilization of specific proteins as an integral component of a chaperone complex. (3-6) Furthermore, HSP90 is highly conserved between species; having 60% and 78% amino acid similarity between mammalian and the corresponding yeast and Drosophila proteins, respectively. HSP90 is a highly conserved and essential stress protein that is expressed in all eukaryotic cells. Despite its label of being a heat-shock protein, HSP90 is one of the most highly expressed proteins in unstressed cells (1–2% of cytosolic protein). It carries out a number of housekeeping functions – including controlling the activity, turnover, and trafficking of a variety of proteins. Most of the HSP90-regulated proteins that have been discovered to date are involved in cell signaling (7-8). The number of proteins now know to interact with HSP90 is about 100. Target proteins include the kinases v-Src, Wee1, and c-Raf, transcriptional regulators such as p53 and steroid receptors, and the polymerases of the hepatitis B virus and telomerase.5 When bound to ATP, HSP90 interacts with co-chaperones Cdc37, p23, and an assortment of immunophilin-like proteins, forming a complex that stabilizes and protects target proteins from proteasomal degradation. In most cases, HSP90-interacting proteins have been shown to co-precipitate with HSP90 when carrying out immune adsorption studies, and to exist in cytosolic heterocomplexes with it. In a number of cases, variations in HSP90 expression or HSP90 mutation has been shown to degrade signaling function via the protein or to impair a specific function of the protein (such as steroid binding, kinase activity) in vivo. Ansamycin antibiotics, such as geldanamycin and radicicol, inhibit HSP90 function (9). Recently, Prof. Tatu's laboratory has shown the importance of HSP90 in parasite growth. They have shown that inhibition of P. falciparum HSP90 (PfHSP90), blocks the erythrocytic cycle by inhibiting stage transformation, leading to inhibition of parasite growth (10, 11). Looking for more information on HSP90? Visit our new HSP90 Scientific Resource Guide at http://www.HSP90.ca.
REFERENCES
1. Nemoto T., et al. (1997) J. Biol Chem. 272: 26179-26187.
2. Minami Y., et al. (1991) J. Biol Chem. 266: 10099-10103.
3. Arlander S.J.H., et al. (2003) J. Biol Chem. 278: 52572-52577.
4. Pearl H., et al. (2001) Adv Protein Chem. 59: 157-186.
5. Neckers L., et al. (2002) Trends Mol Med. 8: S55-S61.
6. Pratt W., Toft D. (2003) Exp Biol Med. 228: 111-133.
7. Pratt W., Toft D. (1997) Endocr Rev. 18: 306–360.
8. Pratt W.B. (1998) Proc Soc Exptl Biol Med. 217: 420–434.
9. Whitesell L., et al. (1994) Proc Natl Acad Sci USA. 91: 8324–8328.
10. Banumathy G., Singh V., Pavithra S.R., and Tatu U. (2003) J Biol Chem. 278(20): 18336-45.
11. Pavithra S.R, Banumathy G., Joy O., Singh V., and Tatu U. (2004) J Biol Chem. 279(45):46692-9.
RESEARCH AREA
Cancer | Heat Shock | Cell Signaling | Protein Trafficking | Chaperone Proteins | Cancer | Tumor Biomarkers
ALTERNATIVE NAMES
PF14_0417 HSP90 Antibody, Heat shock 86 kDa antibody, heat shock 90kDa protein 1 alpha antibody, Heat shock protein 90kDa alpha cytosolic class A member 1 antibody, Heat shock protein 90kDa alpha cytosolic class B member 1 antibody, Heat shock protein HSP 90 alpha antibody, Heat shock protein HSP 90 beta antibody, Heat shock protein HSP 90-alpha antibody, HSP 84 antibody, HSP 86 antibody, HSP 90 antibody, HSP90 Beta antibody, HSP90A antibody, HSP90AA1 antibody, HSP90AB1 antibody, HSP90B antibody, HSP90N antibody, HSPC1 antibody, HSPC2 antibody, HSPCA antibody, HSPCAL1 antibody, HSPCAL4 antibody, HSPCB antibody, HSPN antibody